Navigating cTTP
Long-term morbidity:
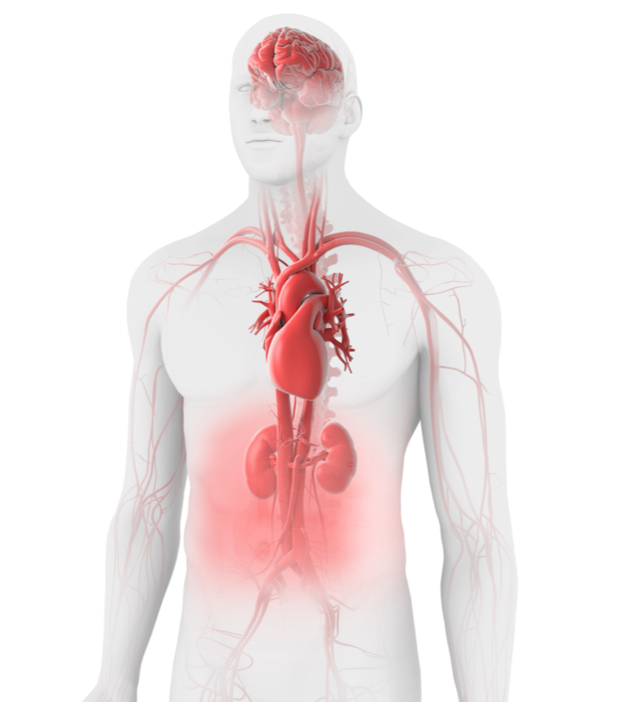
cTTP can affect many organs and systems, and can cause neurologic symptoms (like confusion, headache, focal neurological deficits, paresis, speech impairment, visual changes, encephalopathy, coma, and depression), cardiovascular symptoms (like chest pain, heart failure, hypotension, and stroke/transient ischemic attack), gastrointestinal symptoms (like abdominal pain), and/or renal symptoms (like proteinuria and microhematuria), in addition to fever and purpura, which appears as purplish bruises or pinpoint-sized dots on skin or mucous membranes.4,9
TTP manifests as acute events with short- and long-term outcomes and is associated with a significant disease burden.4,5 Quality of life and lifespan are significantly reduced compared to the general population due to serious, ongoing widespread organ damage and other comorbidities resulting from an ADAMTS13-deficient state.
Patients’ quality of life also suffers due to the treatment burden and complications.10
Mortality:
cTTP is associated with a 90% mortality rate if left untreated and even with treatment, the mortality rate remains high (5-16%) especially during acute episodes.9,11